American experts that three genes affected by similar aspects of neuronal formation. What impact does the finding have?
Autism spectrum disorder remains a mystery to modern medicine. Lately, it has been associated with hundreds of different genes, but it remains strange how these different gene mutations converge on similar pathology in patients.
Now, researchers from Harvard University and the Broad Institute of MIT and Harvard have discovered that three different autism risk genes affect similar aspects of neuronal formation and the same types of neurons in the developing human brain. By testing the gene mutations in miniature 3D models of the human brain called “brain organoids,” the researchers identified similar general defects for each risk gene, although each acted through unique underlying molecular mechanisms.
The results, published in the journal Nature, give researchers a better understanding of autism spectrum disorder and are a first step in finding treatments for the condition.
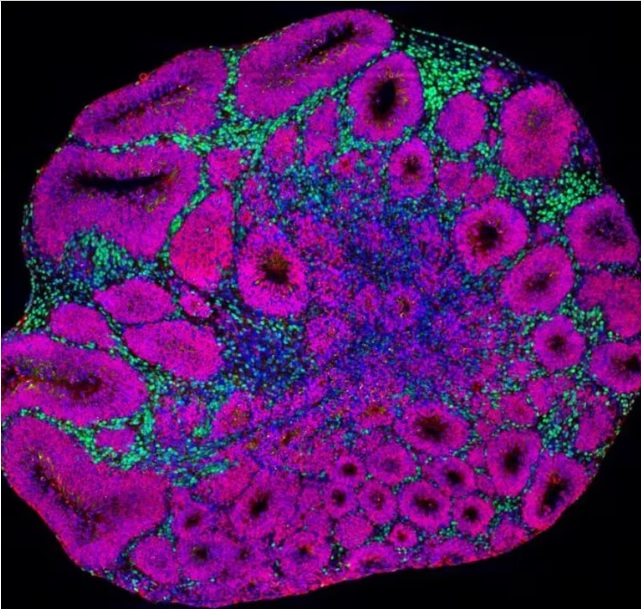
Microscopy image of a brain organoid showing neuron precursors (magenta) and deep-layer projection neurons (green), which are one of the cell types affected by autism risk gene mutations. (Credit: Paola Arlotta lab at Harvard University and Kwanghun Chung lab at MIT).
“A lot of effort in the field is devoted to understanding whether there are commonalities among the many risk genes associated with autism. Finding such shared features may highlight common goals for broad therapeutic intervention, regardless of the genetic origin of the disease. Our data show that, in fact, multiple disease mutations converge to affect the same cells and developmental processes, but through different mechanisms. These results encourage future investigation of therapeutic approaches directed at modulating shared dysfunctional brain properties,” said study lead author Paola Arlotta, Golub Family Professor of Stem Cells and Regenerative Biology at Harvard University and a member of the institute at the Stanley Center for Psychiatric Research at the Broad Institute.
The expert focuses on organoid models of the human cerebral cortex, the part of the brain responsible for cognition, perception and language. The models start out as stem cells, then grow into a 3D tissue containing many of the cell types of the cortex, including neurons that can fire and connect to circuits. “In 2019, we published a method to enable the production of organoids with the unique ability to grow reproducibly. They consistently form the same cell types, in the same order, as the developing human cerebral cortex,” said Silvia Velasco, a senior postdoctoral fellow in Arlotta’s lab and co-lead author of the new study. “It is a dream come true to now see that organoids can be used to discover something unexpected and very new about a disease as complex as autism.”
In the new study, the researchers generated organoids with a mutation in one of three autism risk genes, called SUV20H1, ARID1B and CHD8. “We decided to start with three genes that have a very broad hypothetical function. They don’t have a clear function that can easily explain what happens in autism spectrum disorder, so we were interested to see if these genes were doing similar things in any way,” said Bruna Paulsen, a postdoctoral fellow in Arlotta’s lab and collaborator. primary author.
The researchers then grew the organoids over the course of several months, closely modeling the progressive stages of how the human cerebral cortex forms. And they analyzed the organoids using several technologies: single-cell RNA sequencing and single-cell ATAC sequencing to measure changes and regulation in gene expression caused by each disease mutation; proteomics to measure protein responses; and calcium images to verify if the molecular changes were reflected in the abnormal activity of the neurons and their networks.
“This study was only possible as a collaboration of several laboratories coming together, each with their own expertise, to attack a complex problem from multiple angles,” said co-author Joshua Levin, an institute scientist at the Stanley and Klarman Cell Center and from the Broad Institute Observatory. The researchers found that all of the risk genes affected neurons in similar ways, either speeding up or slowing down neuronal development. In other words, the neurons developed at the wrong time. Also, not all cells were affected; rather, all of the risk genes affected the same two populations of neurons, an inhibitory type called GABAergic neurons and an excitatory type called deep-layer excitatory projection neurons. This targeted select cells that may be special targets in autism.
“The cortex is made in a very orchestrated way: each type of neuron appears at a specific time and they start to connect very early. If some cells form too early or too late compared to what they are supposed to, it may be changing how circuits are ultimately wired,” said Martina Pigoni, a former postdoctoral fellow in the lab of Arlotta and co-lead author.
In addition to testing different risk genes, the researchers also produced organoids using stem cells from different donors. “Our goal was to see how changes in organoids might be affected by an individual’s unique genetic background,” said Amanda Kedaigle, a computational biologist in Arlotta’s lab and co-senior author. Looking at organoids made from different donors, the overall changes in neural development were similar, but the level of severity varied between individuals. The effects of risk genes were adjusted for the rest of the donor genome.
“It is puzzling how the same autism risk gene mutations often show variable clinical manifestations in patients. We found that different human genomic contexts can modulate the manifestation of disease phenotypes in organoids, suggesting that we may use organoids in the future to unravel these distinct genetic contributions and move closer to a more comprehensive understanding of this complex pathology,” Arlotta. said.
“Genetic studies have been highly successful in identifying alterations in the genome associated with autism spectrum disorders and other neurodevelopmental conditions. The next difficult step on the road to the discovery of new treatments is to understand exactly what these mutations do to the developing brain,” said Steven Hyman, who is a professor in the Distinguished Service of Stem Cells and Regenerative Biology at Harvard University, director of the Stanley Center on the Broad, and a Senior Fellow of the Broad Institute.
“By mapping the alterations in brain circuits when there are genetic variations, we can take the next tentative step in the direction of better diagnostics and discover new avenues for therapeutic exploration,” the experts summarized in the study.